When you pick up a prescription, you might see two names on the bottle: one you recognize, like Lipitor or Nexium, and another that looks completely different, like atorvastatin or esomeprazole. The second one is a generic drug. It’s not a copy, knockoff, or lower-quality version. It’s the exact same medicine, approved by the FDA to work just as well as the brand-name version - but at a fraction of the cost.
What Exactly Is a Generic Drug?
A generic drug is a pharmaceutical product that contains the same active ingredient, in the same strength, and delivered the same way as a brand-name drug. It’s not a similar version. It’s not a close match. It’s identical in how it works inside your body. The FDA defines it clearly: a generic drug must be bioequivalent to its brand-name counterpart. That means it delivers the same amount of medicine into your bloodstream at the same rate. If you take a generic version of metformin for diabetes, your body processes it exactly as it would the brand-name Glucophage. The key difference isn’t in the medicine itself - it’s in the packaging, the name, and the price. Generic drugs become available after the original patent on the brand-name drug expires. Patents typically last 20 years from the date they’re filed. Once that window closes, other manufacturers can apply to make the same drug under its chemical name.How Are Generic Drugs Approved?
Getting a generic drug onto the market doesn’t require repeating all the expensive clinical trials that the original company went through. Instead, manufacturers use a streamlined process called the Abbreviated New Drug Application (ANDA). This system was created by the Hatch-Waxman Act of 1984, which balanced the need for innovation with the need for affordable medicine. To get FDA approval, a generic drug maker must prove three things:- Pharmaceutical equivalence: The generic has the same active ingredient, strength, dosage form (tablet, capsule, liquid), and route of administration (oral, injection, etc.) as the brand-name drug.
- Bioequivalence: The generic must deliver the same amount of active ingredient into your bloodstream within the same timeframe. The FDA requires that the 90% confidence interval for the ratio of the generic’s AUC (area under the curve) and Cmax (peak concentration) falls between 80% and 125% of the brand-name drug. In plain terms: your body absorbs it the same way.
- Manufacturing quality: The facility where the generic is made must meet the same Current Good Manufacturing Practices (cGMP) as the brand-name maker. The FDA inspects over 3,500 generic drug plants worldwide each year - the same number as brand-name facilities.
Are Generic Drugs Really as Effective?
Yes. And the evidence is overwhelming. The FDA doesn’t approve a generic unless it performs the same as the brand-name drug in real-world conditions. Multiple studies back this up. In 2010, the Institute of Medicine reviewed 38 clinical trials on generic cardiovascular drugs and found no meaningful difference in effectiveness or safety compared to brand-name versions. The American College of Physicians, the American Medical Association, and the CDC all recommend using generics when available. Dr. Janet Woodcock, former director of the FDA’s drug evaluation center, put it plainly: “The FDA would not allow generics to be marketed unless they were therapeutically equivalent to the brand.” There are rare exceptions. Some drugs have a narrow therapeutic index - meaning even a small change in blood levels can cause problems. Examples include warfarin (a blood thinner), levothyroxine (for thyroid conditions), and certain anti-seizure medications. In these cases, doctors may prefer to stick with one version, brand or generic, to avoid any potential fluctuation. But even here, switching isn’t dangerous - it just requires closer monitoring.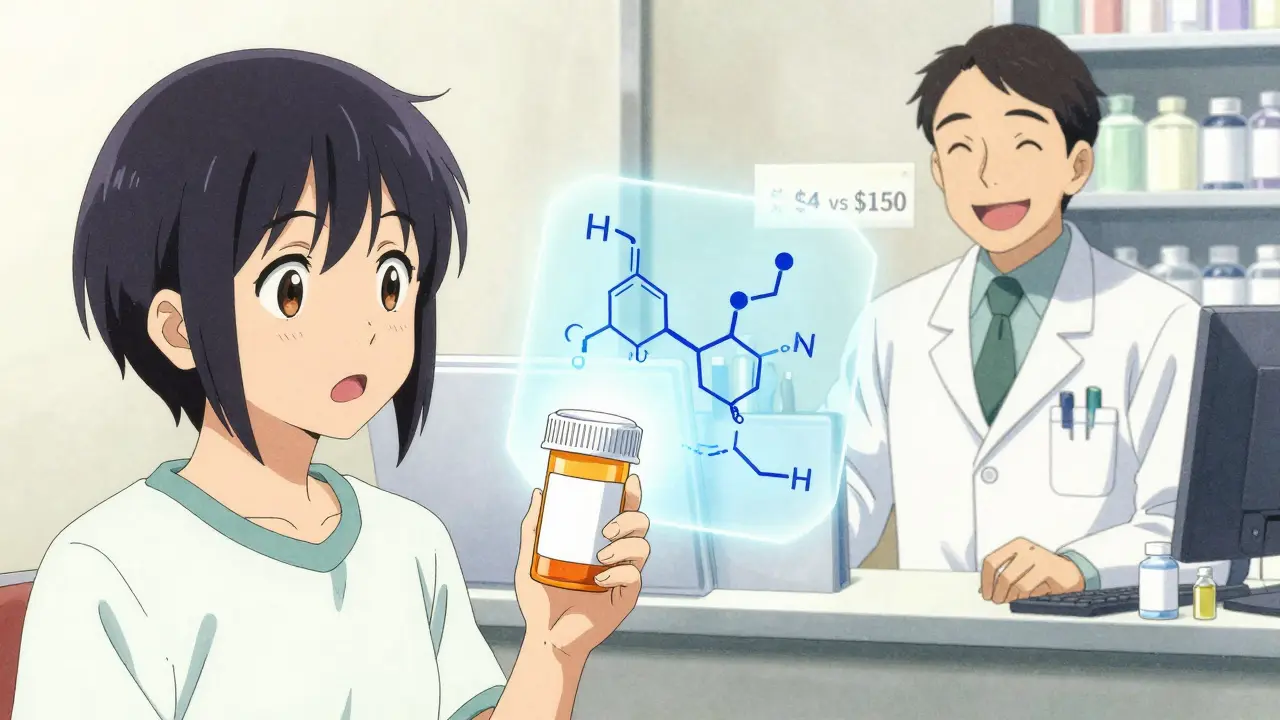
How Much Do Generic Drugs Save?
The savings are massive. Generic drugs account for 90% of all prescriptions filled in the U.S., but they make up only about 13% of total drug spending. That means generics saved the U.S. healthcare system over $2 trillion in the last decade alone. On average, a generic drug costs 80-85% less than its brand-name equivalent. In some cases, when five or more companies start making the same generic, prices can drop to just 9% of the original brand price. The reason? No one has to pay for the original $2.6 billion in research and development costs. Generic manufacturers only need to prove bioequivalence - a process that costs a few hundred thousand dollars, not billions. This isn’t just about big savings for insurance companies. It’s about real people. A patient on a generic version of lisinopril for high blood pressure might pay $4 a month instead of $150. A child on generic amoxicillin might get the full course for under $10 instead of $80. These aren’t hypotheticals - they’re everyday realities.What About Biosimilars?
Not all drugs are created equal when it comes to generics. Traditional generics are made from small-molecule chemicals - simple, stable compounds that are easy to copy exactly. But biologics - complex drugs made from living cells - are a different story. These include drugs for rheumatoid arthritis, cancer, and diabetes like Humira, Enbrel, and insulin. Because biologics are made from living organisms, they can’t be copied exactly. Instead, manufacturers create “biosimilars” - drugs that are highly similar to the original, with no clinically meaningful differences. The approval process for biosimilars is more complex and expensive than for traditional generics. As a result, they typically cost only 20-30% less than the brand-name biologic, not 80-85%. Still, they’re a major step forward in making these life-saving drugs more affordable.
Why Don’t More People Use Generics?
Many patients still believe generics are inferior. Some doctors hesitate to prescribe them. Pharmacists sometimes get calls from patients who refuse to take a pill because it looks different. But these fears aren’t based on science. The FDA requires identical labeling for generics and brand-name drugs - except for the brand name and manufacturer. The patient information leaflet is the same. The warnings, side effects, and usage instructions are unchanged. The only difference is the price tag. One reason for confusion is the lack of transparency. If your doctor writes “Lipitor” on your prescription, the pharmacy may automatically fill it with atorvastatin unless you specifically ask for the brand. But if you’re not told why, you might assume the change is a cost-cutting move - not a medically sound substitution.What’s Changing in the Generic Drug Market?
The landscape is shifting. Over 350 brand-name drugs with $138 billion in annual sales are set to lose patent protection between 2023 and 2027. That means a wave of new generics is coming - for cholesterol drugs, asthma inhalers, and even some cancer treatments. But there are challenges. About 80% of the active ingredients in generic drugs come from facilities in India and China. Supply chain disruptions, like those seen during the pandemic, can lead to shortages. The FDA reported a 22% increase in drug shortages in 2022, partly due to quality issues at overseas plants. New rules are helping. The FDA’s Generic Drug User Fee Amendments (GDUFA) III, which took effect in 2022, aims to cut approval times to 10 months for 90% of applications. The CREATES Act of 2019 also helps prevent brand-name companies from blocking generic entry by withholding samples needed for testing. Another trend? Authorized generics. These are brand-name drugs sold under a generic label by the original manufacturer - often at a lower price. They’re not a loophole; they’re a strategy to compete with other generics before the patent fully expires.What Should You Do?
If you’re prescribed a medication, ask your doctor or pharmacist: “Is there a generic version?” If there is, it’s almost always the better choice - unless your doctor has a specific reason not to switch. Don’t assume a higher price means better quality. Don’t refuse a pill because it looks different. And don’t let confusion cost you money. Generics aren’t a compromise. They’re the standard. The system works. The science is solid. The savings are real. And if you’re taking a generic drug right now, you’re already benefiting from one of the most successful public health innovations in modern medicine.Are generic drugs as safe as brand-name drugs?
Yes. Generic drugs must meet the same FDA standards for safety, strength, purity, and quality as brand-name drugs. The FDA requires that generics be bioequivalent - meaning they work the same way in your body. They’re held to identical manufacturing standards and undergo the same post-market monitoring for side effects and safety issues.
Why do generic pills look different from brand-name pills?
Trademark laws require generic drugs to look different from the brand-name version. This includes color, shape, size, and markings. But these differences are only in the inactive ingredients - like dyes or fillers - which don’t affect how the medicine works. The active ingredient, strength, and performance are identical.
Can I switch between brand-name and generic drugs safely?
For most medications, yes. For drugs with a narrow therapeutic index - like warfarin, levothyroxine, or certain seizure medications - your doctor may recommend sticking with one version to avoid small changes in blood levels. But switching isn’t dangerous. It just requires more careful monitoring. Always talk to your doctor before switching.
Do generic drugs take longer to work?
No. Generic drugs must demonstrate bioequivalence - meaning they reach the same peak concentration in your bloodstream at the same time as the brand-name version. There’s no delay in how quickly they start working. Any perceived difference is usually due to placebo effect or unrelated factors like diet or other medications.
Why are generic drugs so much cheaper?
Generic manufacturers don’t have to repeat the expensive clinical trials that brand-name companies do. The original company spends an average of $2.6 billion to develop a new drug. Generic makers only need to prove their version works the same way - which costs a fraction of that. Competition among multiple generic manufacturers drives prices even lower.
Are all generic drugs made in the U.S.?
No. About 80% of the active ingredients in generic drugs come from facilities in India and China. However, all manufacturing sites - whether in the U.S., India, or elsewhere - must meet the same FDA standards. The FDA inspects these facilities just as rigorously as U.S.-based plants.
Can pharmacists substitute a generic without my doctor’s permission?
In 49 U.S. states, yes - unless your doctor writes “dispense as written” on the prescription. Pharmacists are legally allowed to substitute a generic if it’s approved as therapeutically equivalent. This is standard practice and helps reduce costs. You can always ask if a generic is available and whether it’s right for you.









Comments
Oh wow, another FDA propaganda piece. You really believe these generics are identical? Let me tell you about my cousin who switched to generic levothyroxine and ended up in the ER with a heart rate of 160. The fillers? They’re not inert. They’re Chinese chalk and industrial dye. The FDA inspects plants? Sure, they inspect the ones they’re told about. The rest? Hidden in back-alley labs with no oversight. You think your $4 pill is safe? It’s a gamble with your life.
How quaint. You treat bioequivalence like it’s gospel. The 80-125% range isn’t precision-it’s a legal loophole dressed up as science. If your AUC dips below 80%, you’re underdosed. If it spikes above 125%, you’re overdosed. That’s not identical. That’s a range wide enough to drive a truck through. And don’t get me started on the fact that most generics are manufactured in countries where regulatory capture is the norm. You’re not saving money-you’re outsourcing risk.
Generic drugs are therapeutically equivalent to brand-name drugs per FDA standards. This is a well-documented fact supported by clinical evidence and regulatory oversight.
Thank you for this. I’ve been telling my patients for years that generics aren’t second-rate-they’re science-made affordable. I had a diabetic patient who was skipping doses because Glucophage cost $120 a month. Switched her to metformin. She’s been stable for 3 years now. No side effects, no complaints. The pill looks different? So what. Her HbA1c didn’t change. That’s what matters.
And to the folks worried about fillers-your body doesn’t care if it’s cornstarch or microcrystalline cellulose. It only cares about the active ingredient. And that’s identical. Please, stop letting fear drive your health decisions.
This is one of those rare moments where capitalism actually works for the people. Imagine if every medicine stayed at $150 a bottle forever just because some corporation got a 20-year monopoly on a molecule. We’d all be broke or dead. Generics are the quiet revolution-the unsung heroes of American healthcare. That $4 pill? That’s the difference between taking your meds and not. Between living and just existing. Keep fighting for access. Keep choosing the science over the brand name.
👏👏👏 finally someone says it right. In India, generics are life. My aunt got cancer treatment for 1/10th the US price. Same drug. Same results. The system works. 💯
Actually, you’re all wrong. The FDA doesn’t require generics to be bioequivalent in all patient populations. There’s a documented bias in clinical trials-they’re done on young, healthy white males. What about elderly patients with liver disease? Or people of color with different metabolisms? The 80-125% range ignores pharmacogenomics entirely. This isn’t science-it’s a corporate-friendly myth.
Let’s be honest: the entire generic drug system is a sham. The FDA’s approval process is a rubber stamp. Most of these facilities are inspected once every five years, if that. And the fact that 80% of active ingredients come from China and India? That’s not supply chain-it’s strategic vulnerability. One geopolitical incident, one quality control failure, and millions of Americans are suddenly without essential meds. This isn’t affordable healthcare-it’s a ticking time bomb disguised as a cost-saving measure.
Interesting how you gloss over the fact that 70% of generic drug shortages between 2018-2022 were due to manufacturing failures at overseas facilities. You call it ‘the same standard’-but the FDA’s own reports show 40% of foreign plants have serious cGMP violations. Your $4 pill might be safe today. But next month? Maybe not. And who’s to blame? The consumer. You’re the one choosing the cheaper option. Don’t act surprised when it fails.
Generics? Sure. But don’t pretend this is about health. It’s about control. The government lets Big Pharma lock up patents, then when the patent dies, they hand the keys to the lowest bidder. Who wins? The Chinese state-owned factories. Who loses? The American worker. The real scandal isn’t the price-it’s the fact that we’ve outsourced our medicine to authoritarian regimes. You want cheap? Fine. But don’t call it progress. Call it surrender.
There is a deeper philosophical truth here: the essence of a medicine lies not in its branding, but in its molecular structure. A molecule of atorvastatin is identical whether it is manufactured in New Jersey or Hyderabad. The human body does not recognize trademarks. It responds only to chemistry. The fear of generics stems not from science, but from the cultural mythology of branding-where we assign value to labels rather than function. To reject a generic is to worship the packaging, not the cure.
And yet, the system is not perfect. The global supply chain is fragile. The FDA is under-resourced. The real challenge is not whether generics work-but whether we have the collective will to fund the oversight that makes them safe. Science has answered the question. Now politics must answer the next one.
My grandma switched to generic lisinopril last year. She was terrified because the pill was blue instead of white. She cried when she saw it. I sat with her and showed her the FDA’s equivalence chart. She took it. Two months later, she said, ‘I feel the same. Why did I worry so much?’
Turns out, the real enemy isn’t the generic. It’s the fear we’re sold. Thanks for reminding us that science doesn’t care about logos.
While the statistical data presented is largely accurate, one must not overlook the epistemological limitations of bioequivalence testing. The FDA’s 80-125% confidence interval is a regulatory convenience, not a biological absolute. Variability in absorption, distribution, metabolism, and excretion across diverse physiological phenotypes renders the notion of ‘identical’ therapeutics a statistically convenient fiction. The assumption that pharmacokinetic equivalence equates to clinical equivalence is a fallacy rooted in reductionist pharmacology. One cannot equate population-level metrics with individual outcomes. The patient is not a data point.