When you hear the word biosimilar, you might think it’s just a cheaper copy of a biologic drug-like how generic pills are exact copies of brand-name ones. But that’s not true. Biosimilars aren’t chemically identical. They’re made from living cells, not synthesized in a lab. And because of that, tiny differences in how they’re built can trigger your immune system in ways the original drug doesn’t. That’s immunogenicity-and it’s the biggest unanswered question about biosimilars.
What Immunogenicity Really Means
Immunogenicity is when your body sees a drug as a threat and makes antibodies against it. These are called anti-drug antibodies, or ADAs. For some biologics, up to 70% of patients develop them over time. That doesn’t mean everyone gets sick. But if those antibodies neutralize the drug-block it from working-you could lose control of your disease. In rare cases, they can cause serious reactions like anaphylaxis. The drug cetuximab, for example, triggered life-threatening allergies in some patients because of a sugar molecule (galactose-α-1,3-galactose) attached to it during production.Biosimilars are designed to be as close as possible to the original biologic. But because they’re made in living cells-usually Chinese hamster ovary cells or human cell lines-small changes in sugar attachments, protein folding, or even tiny impurities can happen. These aren’t mistakes. They’re unavoidable in biological manufacturing. The question isn’t whether differences exist. It’s whether they matter.
Why Biosimilars Can Trigger Different Immune Responses
Your immune system doesn’t care if a drug is called "biosimilar" or "originator." It only cares about what it sees on the molecular level. Here’s what can make a difference:- Post-translational modifications: These are chemical changes made to proteins after they’re made. Glycosylation-adding sugar groups-is the most common. Even a 5% difference in sialic acid or galactose content can change how your immune cells recognize the drug.
- Protein aggregates: If even 5% of the drug forms clumps, immunogenicity risk jumps 3.2 times. These clumps look like invaders to your immune system.
- Host cell proteins: Tiny amounts of leftover proteins from the manufacturing cells (over 100 ppm) can act as immune triggers. One study found these were linked to 87% higher ADA rates.
- Stabilizers: The biosimilar version of rituximab uses polysorbate 80. The original uses polysorbate 20. These are similar, but not the same. One might cause more protein instability, leading to more clumping-and more immune reactions.
These aren’t theoretical concerns. They’re measurable. And they matter more in some patients than others.
Who’s Most at Risk?
Not everyone reacts the same way. Your immune system’s sensitivity depends on several things:- Disease state: People with rheumatoid arthritis have 2.3 times higher risk of developing ADAs than healthy people. Their immune systems are already on high alert.
- Genetics: Certain HLA gene variants-like HLA-DRB1*04:01-can increase ADA risk by nearly fivefold for some drugs.
- Medications you’re on: Methotrexate, commonly used with biologics, cuts ADA rates by 65%. It’s not just a painkiller-it’s an immune modulator.
- How you get the drug: Injecting under the skin (subcutaneous) is 30-50% more likely to cause immune responses than IV infusion. That’s why some biosimilars are designed for injection-they’re more exposed to immune cells in the skin.
- Duration of treatment: Your body doesn’t react immediately. It takes months of repeated exposure before tolerance breaks. After six months, ADA risk climbs steadily.
And here’s the twist: immunocompromised patients-like those on chemotherapy or high-dose steroids-often have lower ADA rates. Their immune systems are too suppressed to mount a strong response. But that doesn’t mean they’re safer. It just means the risk profile changes.
How Do We Measure This?
Testing for ADAs isn’t like checking blood sugar. It’s complicated. Labs use different tools, and results vary wildly.- Screening assays: Usually ELISA or electrochemiluminescence (ECL). ECL is more sensitive-it finds ADAs in up to 13.1% of patients, while older methods might miss half of them.
- Confirmatory assays: These check if the antibody actually binds to the drug, not just random proteins.
- Neutralizing antibody tests: The gold standard. These use live cells to see if the antibody blocks the drug’s function. But they’re messy. Results can vary by 25-30% between labs.
Here’s the problem: if one study uses ECL and another uses ELISA, you can’t compare their results. That’s why regulators like the FDA and EMA insist that biosimilar trials must use the exact same testing methods as the reference product. Otherwise, you’re not comparing drugs-you’re comparing tools.
What Does Real-World Data Show?
The data is mixed. Some studies show no difference. Others show small but real gaps.- The NOR-SWITCH trial (2016) found slightly higher ADA rates in patients switched to the infliximab biosimilar (11.2% vs. 8.5%), but no drop in effectiveness or increase in side effects.
- A 2021 study of 1,247 rheumatoid arthritis patients found nearly identical ADA rates between reference infliximab and its biosimilar CT-P13 (12.3% vs. 11.8%).
- But in Denmark, a registry study found adalimumab biosimilars had higher ADA rates (23.4%) than Humira (18.7%)-and the difference was statistically significant (p=0.03). Yet, clinical outcomes stayed the same.
On Reddit, patients report both sides: some say switching caused severe injection site reactions. Others say they felt no difference after years on biosimilars. One rheumatologist survey found 68% of doctors think immunogenicity fears are overblown. But 22% have seen real, clinically relevant differences in practice.
The takeaway? For most people, biosimilars work just as well. But for a small group-maybe 5-10%-the immune system notices the difference. And when it does, it can change the game.
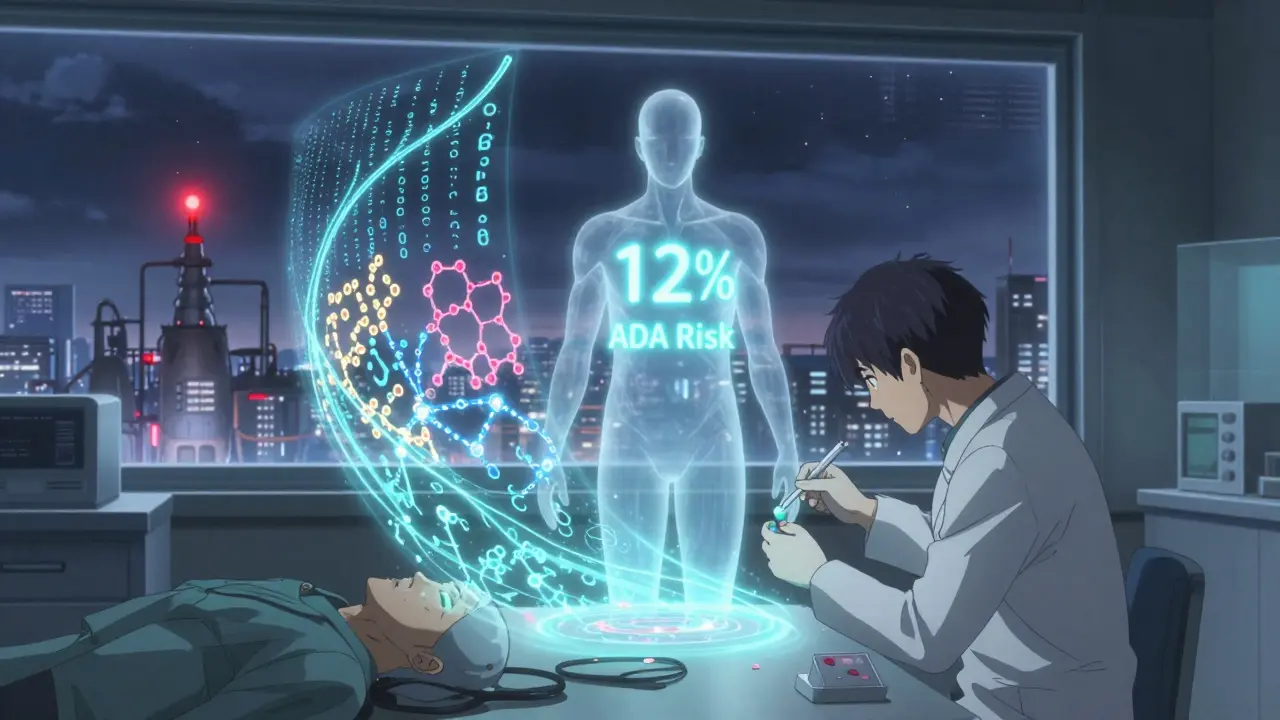
Regulators Are Watching Closely
The FDA and EMA don’t accept "close enough." They demand a "totality of evidence"-analytical data, animal studies, clinical trials-all focused on proving similarity. Immunogenicity is a mandatory endpoint. Biosimilar makers must run head-to-head trials using identical assays. No shortcuts.And they’re getting better. New tools like advanced mass spectrometry can now map protein structures with 99.5% accuracy. By 2027, experts predict we’ll be able to spot glycosylation differences before the drug even leaves the factory. That’s huge.
But the FDA’s Dr. Rina Singh warns: even tiny changes in the Fc region-below 5%-can alter how the drug interacts with immune cells. In susceptible people, that’s enough to trigger a response.
The Future: Multi-Omics and Personalized Risk
The next big leap isn’t just better manufacturing. It’s better prediction.- Proteomics: Mapping every protein in the drug.
- Glycomics: Analyzing every sugar group attached.
- Immunomics: Studying how your immune genes respond to specific drug shapes.
Researchers at UCSF and other centers are already combining these into risk profiles. Imagine a blood test before you start a biosimilar that says: "Your HLA type and immune history suggest a 12% risk of ADA development with this product." That’s not science fiction. It’s coming.
Right now, the system works because most patients don’t react. But for those who do, the difference between originator and biosimilar can be real-even if it’s not always clinical.
What Should You Do?
If you’re on a biologic and considering switching to a biosimilar:- Don’t panic. Most people switch without issue.
- Ask your doctor if your current drug has a known immunogenicity risk. Some, like TNF inhibitors, are more likely to trigger ADAs than others.
- If you’ve had side effects before, keep a symptom journal. Note injection site reactions, fatigue, or flare-ups.
- Don’t assume biosimilar = always safer or always riskier. It’s about the product, not the label.
- Ask if your clinic uses standardized ADA testing. If they don’t, it’s harder to track changes.
For now, biosimilars are a win-cheaper, widely available, and effective for millions. But immunogenicity isn’t a bug. It’s a feature of biological complexity. And until we can predict who will react, we have to treat each switch as a personal experiment-not a guaranteed swap.
Are biosimilars the same as generics?
No. Generics are exact chemical copies of small-molecule drugs, like aspirin or metformin. Biosimilars are made from living cells and are "highly similar" to biologics, but not identical. Tiny differences in structure can affect how your immune system reacts.
Can biosimilars cause more side effects than the original drug?
In most cases, no. Large studies show similar safety profiles. But for a small subset of patients, biosimilars may trigger slightly higher rates of anti-drug antibodies, which can lead to injection site reactions, reduced effectiveness, or rare allergic responses. It’s not common, but it’s possible.
Why do some patients react to biosimilars but not the original drug?
Minor differences in manufacturing-like sugar attachments, protein clumps, or stabilizers-can create new immune triggers. Your body may have tolerated the original drug’s exact shape, but the biosimilar’s slightly different structure could be recognized as foreign. It’s like recognizing a face with a new hairstyle versus a completely different person.
Is immunogenicity testing routine for biosimilars?
Yes. Regulatory agencies require biosimilar manufacturers to test immunogenicity head-to-head with the reference product using identical methods. But routine testing for patients isn’t standard yet. It’s usually only done in research settings or if a patient loses response to treatment.
Should I avoid switching to a biosimilar because of immunogenicity?
For most people, the benefits outweigh the risks. Biosimilars are significantly cheaper and have proven effective for millions. If you’re doing well on your current drug, discuss the switch with your doctor. If you’ve had immune-related side effects before, ask about monitoring options. Avoiding a biosimilar out of fear may cost you more in long-term expenses-and limit access to treatment.





Comments
The notion that biosimilars are 'just cheaper copies' is a dangerous oversimplification. The glycosylation profiles alone-especially sialic acid and galactose heterogeneity-can shift the FcγRIIIa binding affinity by up to 40%, altering ADCC activity. This isn't theoretical; it's quantifiable via LC-MS/MS. If your lab is still using ELISA for ADA detection, you're operating in the dark ages. ECL is the baseline now, and even that has inter-lab variability north of 25%. The FDA's insistence on identical assay platforms isn't bureaucracy-it's scientific hygiene.
Okay but have you heard what happened to my cousin? She switched to the biosimilar for Humira and got these terrifying hives-like, full-body, epinephrine-level hives. The doctor said it was 'probably coincidence' but I know what I saw. They're hiding the real risks because big pharma wants to save money. And don't even get me started on how they use Chinese hamster cells-those are full of weird proteins no one even tests for. This is like playing Russian roulette with your immune system.
Bro, I've been on a biosimilar for RA for 3 years now. Zero issues. No flares, no rashes, no weird fatigue. My doc said the data shows 9 out of 10 people switch fine. Don't let one scary story scare you. If your body's doing well, stick with it. If you're thinking of switching, talk to your rheum. But don't panic. We've got millions on these now. It's not magic, it's science.
I appreciate how nuanced this post is. It’s easy to fall into binary thinking-either biosimilars are perfect or they’re dangerous. But the reality is layered: most people are fine, a small subset reacts, and we’re still learning why. The key is transparency-patients should know their drug’s manufacturing origin, and clinicians should track ADA trends over time. It’s not about fear. It’s about informed consent.
In India, biosimilars are the only option for most people. We don’t have the luxury of choosing Humira. But here’s the thing-our doctors monitor closely. If someone develops new joint pain or fatigue after switching, we switch back. No drama. No panic. Just data and care. And honestly? Most patients do just fine. The real issue isn’t the drug-it’s access to follow-up care. Without that, even the safest drug becomes risky.
Let’s be clear: the regulatory framework for biosimilars is among the most rigorous in pharmaceutical history. The totality of evidence requirement-analytical, nonclinical, clinical-is not a suggestion. It’s a mandate. And yet, we still see clinicians and patients conflating immunogenicity risk with clinical failure. That’s a communication failure, not a scientific one. The data shows equivalence in efficacy and safety for the vast majority. The outliers? They’re not proof of systemic failure-they’re signals for personalized medicine.
So let me get this straight. You’re telling me that if a biosimilar uses polysorbate 80 instead of 20, and that causes 5% more protein aggregation, and that increases ADA risk by 3.2x, then it’s still considered 'highly similar'? That’s not similar. That’s a different drug with a different name. And yet we call it a biosimilar? The whole system is a shell game.
What’s wild is that we’re still using the same ADA assays from the 2000s. ECL improved sensitivity, sure, but we need multiplexed, single-cell assays to detect low-affinity, non-neutralizing ADAs. And we’re not even talking about T-cell epitope mapping yet. The future isn’t just better manufacturing-it’s better immune profiling. Imagine a blood test that tells you your HLA-DRB1*04:01 status and predicts your ADA risk before you even get the first injection. That’s the real win.
Switching isn't a gamble. It's a decision. Check your lab's testing method. Track symptoms. If you're stable, stay. If you're not, switch back. Simple. No drama. No fear. Just data.
Let’s not sugarcoat this. The entire biosimilar industry is built on the assumption that patients are interchangeable biological units. But we’re not. We have different HLA haplotypes, different gut microbiomes, different immune histories. And yet we treat a 12% ADA difference as statistically insignificant while ignoring the fact that for the person experiencing it, it’s 100% catastrophic. The FDA’s 'totality of evidence' is a legal loophole disguised as science. They’re not proving safety-they’re proving regulatory compliance. Big difference. And don’t get me started on how the industry funds the very studies that 'prove' equivalence. Conflict of interest much?
I’ve been on a biosimilar for 4 years. No issues. My doctor says I’m one of the lucky ones. But I’m grateful. Cheaper drugs mean more people can get treated. I don’t need to know the exact sugar molecule on the protein-I just need it to work. And it does. That’s what matters.
Here’s the real story they don’t tell you: the original biologics were manufactured in the early 2000s with less precise controls. The biosimilars? They’re made with modern tech-cleaner, more consistent. So why would they be *more* immunogenic? It’s not the biosimilar that’s the problem-it’s the original drug’s impurities that were never properly characterized. The FDA is protecting Big Pharma’s legacy products by making biosimilars look riskier. It’s not science. It’s corporate protectionism. And the patients? We’re the ones paying with our immune systems.